COVID-19 (nasal, nasopharyngeal, or throat swab) A&B influenza 2 (nasal or nasopharyngeal swab, also with VTM) RSV (nasopharyngeal swab, also with VTM) Strep A 2 (throat swab)
Detects SARS-CoV-2 in 13 minutes or less5
Leading molecular point-of-care (POC) platform in the United States, trusted by hospitals, physician offices, and urgent care facilities nationwide
Description
Abbott ID NOW is a rapid, instrument-based, isothermal system for the qualitative detection of infectious diseases. The unique ID NOW isothermal nucleic acid amplification technology provides molecular results in just minutes, allowing you to make effective clinical decisions sooner.
“The Abbott ID NOW system combines speed and efficiency for agile delivery of molecular results that can be crucial for faster and more accurate decision-making regarding treatment,” says Arthur Zeraib,
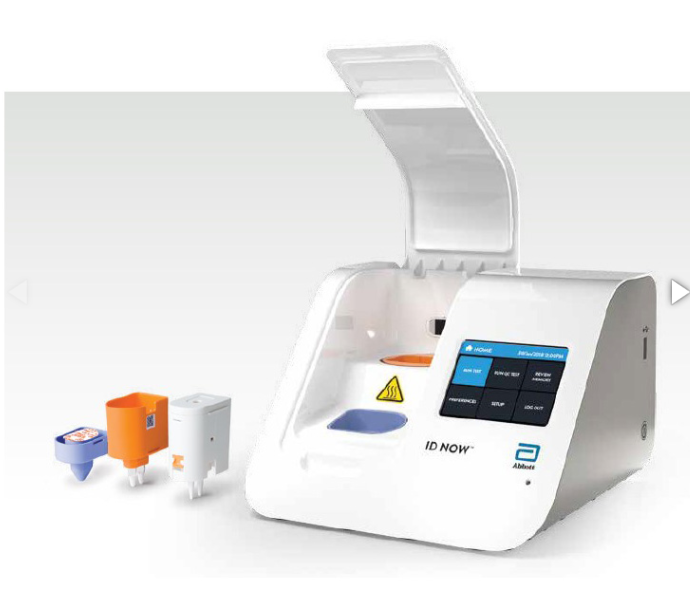
ID NOW (formerly Alere i) combines the benefits of speed and accuracy to deliver lab-accurate results faster than any other molecular method. Small and portable, the ID NOW (formerly Alere i) features an intuitive visual touchscreen, automated on-screen results, color-coded test consumables, and more.

Abbott ID NOW performs best in patients tested early during the disease when the viral load and viral shedding are high (within 1st week of symptom onset). In contrast, the test’s sensitivity decreases during the latter part of the disease (such as in medium to long-term hospitalized patients and patients presenting late in the course of the disease).
Screening COVID-19 with Abbott ID NOW
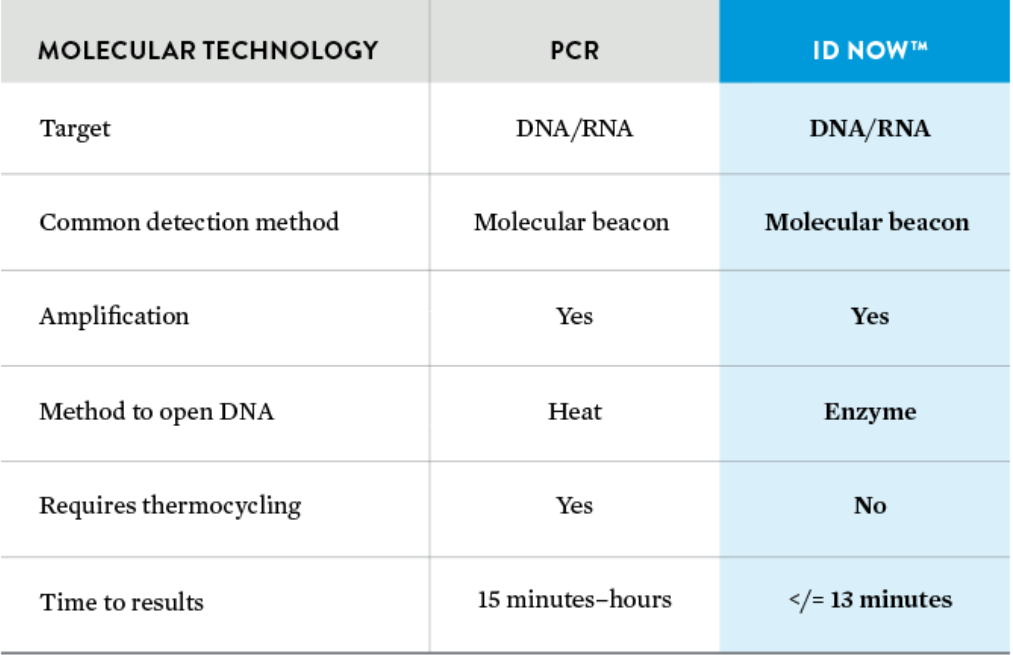
COVID-19 testing with the Abbott ID NOW ™ point-of-care test device offers a cost-effective, resource-efficient, and, most importantly, a rapid alternative to laboratory PCR detection. The principle of the test is the same as that of PCR, but the amplification is carried out using enzymes rather than heat. This significantly speeds up the process so that the result is available in less than 13 minutes instead of several hours. Reliable test results at the lab level and directly at the point of care – with the ID NOW ™, it is now possible!
Abbott ID NOW rapid molecular test for COVID-19 utilizes isothermal amplification of nucleic acid to detect SARS-CoV-2 from nasal swabs and is one of the five platforms BayCare currently uses for COVID-19 testing (in addition to Roche, DiaSorin, BD Max, and Cepheid, which are all RT-PCR assays performed on nasopharyngeal swabs or other specimen types).
The following tests can be performed with the ID NOW:
COVID-19 (nasal, nasopharyngeal, or throat swab)
A&B influenza 2 (nasal or nasopharyngeal swab, also with VTM)
RSV (nasopharyngeal swab, also with VTM)
Strep A 2 (throat swab)
Features:
Leading molecular point-of-care (POC) platform in the United States, trusted by hospitals, physician offices, and urgent care facilities nationwide
High-quality molecular technology targeting COVID-19 RdRp gene
Emergency Use Authorization (EUA) supports flexible near-patient testing environments
Sample types tested direct or in viral transport media (VTM) include: – Throat swab – Nasal swab – Nasopharyngeal swab
ID NOW™ platform designed with POC in mind
Benefits:
Touchscreen displays results, eliminating transcription errors and the need for printing
Its size saves desk space and can be used in any clinical setting
DC lock
Bidirectional connectivity
Delivers exceptional performance
Provides the confidence to make faster clinical decisions
Facilitates effective patient management
Allows rapid introduction of infection control measures
Facilitates specialized antiviral treatments and antimicrobial administration
Reliable Immediate Diagnostic Tests Reduce Overall Healthcare Costs
Overview of the Test
The ID NOW COVID-19 assay is a qualitative, rapid molecular test that utilizes an isothermal nucleic acid amplification technology to detect nucleic acid from the SARS-CoV-2 viral RNA.
Each Abbott ID NOW COVID-19 test cartridge comes with a swab and all the necessary reagents.
The swab included in the test kit is the preferred collection device for optimal tests results. ○ Alternative approved specimen types include direct nasal, nasopharyngeal or oropharyngeal (throat) swabs and nasal, nasopharyngeal, or throat swabs.
Sample to result in time is 13 minutes according to the Instructions for Use.
Each kit contains 24 tests.
Per the Instructions for Use, Abbott requires positive and negative external controls with each new shipment received and once for each untrained operator. The Instructions for Use also state that further controls may be tested to conform with local, state, and federal regulations, accrediting organizations, or your lab’s standard Quality Control procedures.
Negative results should be treated as presumptive and, if inconsistent with clinical signs and symptoms or necessary for patient management, should be tested with different authorized or cleared molecular tests. Negative results do not preclude SARS-CoV-2 infection and should not be used solely for patient management decisions. Negative consequences should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19.
EXTRAORDINARY TECHNOLOGY
ID NOW™ is the fastest point-of-care molecular platform on the market.3
ID NOW™ utilises proven isothermal molecular technology in an intuitive platform, providing the fastest, highly sensitive molecular results in the market at 2 to 13 minutes.4