Clostridium difficile (C. difficile) Influenza A/B Influenza A/B & RSV Methicillin-resistant Staphylococcus aureus SARS-CoV-2 SARS-CoV-2/Flu Assay Strep A
SARS-CoV-2 & Influenza A / B C. difficile Influenza A / B + RSV CT / NG / MG *
Multi- and single-target assays with results in minutes Less than one minute of hands-on time Intuitive, easy-to-use interface with step-by-step guided instruction No operator intervention or interpretation No laptops or additional equipment required
Now there’s a simple, fast and affordable way for you to transfer patient data seamlessly from your Roche cobas Liat PCR System to your EHR with Relaymed, the POC connectivity software solution for the physician office laboratory. Take manual labor and errors out of your workflow today.
Description
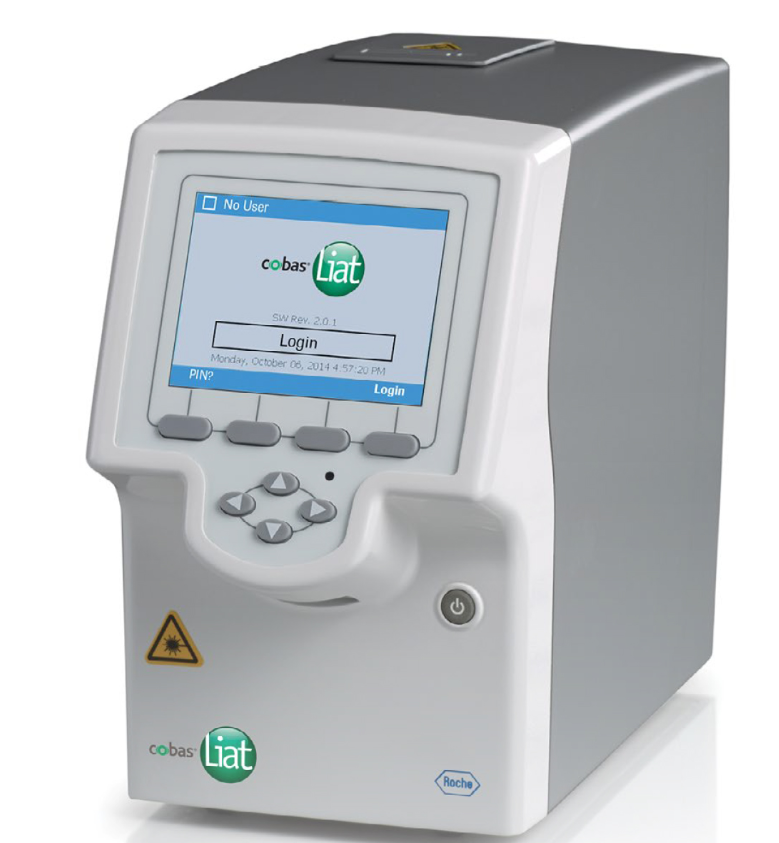
Cobas Liat PCR System is a rapid, point-of-care, RT-PCR test device that can be used to diagnose influenza infection. The system consists of a small bench-top analyzer and a pencil-sized assay tube. It was originally developed by iQuum, and acquired by Roche in 2014. A nasopharyngeal (NP) swab collects a respiratory secretion sample from patients with suspected influenza.
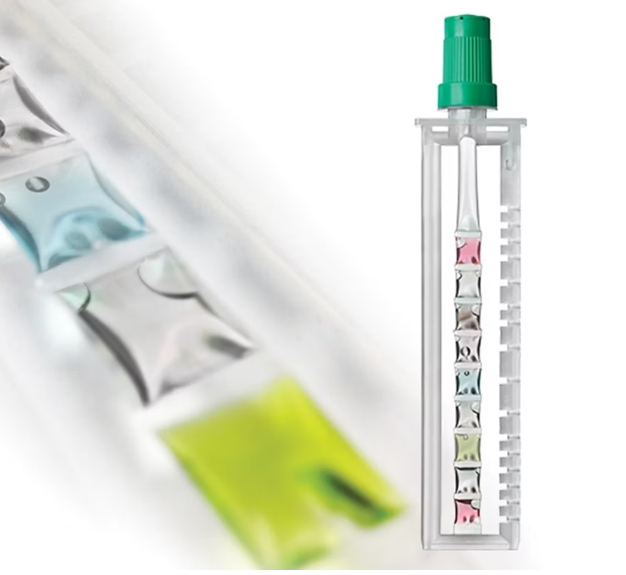
The sample is then transferred by pipette into the single-use assay tube and loaded into the analyzer. It is compressed at different points and times, releasing reagents and moving the sample from one tube segment to another. The presence or absence of influenza A and influenza B is determined using an established nucleic acid assay that detects viral RNA, using RT-PCR. Results are reported in approximately 20 minutes.
Detection of Influenza A and B Viruses and Respiratory Syncytial Virus by Use of Clinical Laboratory Improvement
Cobas Liat was found to have the best performance and agreement compared to the standard RT-PCR for COVID-19 detection. With further research, these rapid tests can be employed in the large-scale screening of COVID-19.
Cobas Liat system SARS-CoV-2 & Influenza A/B nucleic acid test
524 samples were tested with this method. It is an automated multiplex real-time RT-PCR assay for in-vitro qualitative detection and differentiation of SARS-CoV-2, influenza A, and influenza B virus RNA in nasopharyngeal and nasal swabs.
The assay targets both the ORF1 a/b non-structural region and nucleocapsid protein gene that are unique to SARS-CoV-2. An Internal Process Control (IPC) is present to control for adequate processing of the target virus through steps of sample purification, nucleic acid amplification, and to monitor the presence of inhibitors in the RT-PCR processes.
The results are displayed as SARS-CoV-2 detected, not detected, or invalid within 20 minutes. It has got FDA emergency authorization for use.
Cobas Liat System Features:
The Cobas Liat System is a rapid, reverse transcription (RT)-polymerase chain reaction (PCR) point-of-care test that may be as effective as gold standard (lab-based) methods for diagnosing influenza A and influenza B.
Rapid RT-PCR tests for influenza may be better at detecting the presence or absence of influenza than other types of rapid influenza detection tests.
There is limited evidence comparing the Cobas Liat System with other rapid influenza detection tests.
Canadian pricing for the Cobas Liat System is not available.
There is limited evidence for using rapid RT-PCR tests outside a laboratory setting.
Cobas Liat System is a compact, fast, and easy-to-use real-time PCR system designed to test in Point of Care environments, such as primary care practices, pharmacies, and hospital emergency laboratories.
The system includes the cobas® Liat analyzer, a small benchtop analyzer whose smooth operation is ensured by a wide range of internal instrument controls, and which has a growing portfolio of assays, including cobas® Influenza kits. A / B, cobas® Influenza A / B, and RSV, cobas® Strep A and cobas® Clostridium difficile, constantly expanding into new assays for other infectious diseases.
Cobas Liat System Advantage
Small – The analyzer is smaller than a shoebox and is conveniently designed to be placed in tight spaces.
Simple: the cobas® Liat® system is fully automated and has a touch screen that guides the user through the various steps of the technique, requiring minimal training and very little user manipulation time.
Fast – Show results in 20 minutes or less. This allows individual tests to be carried out, and urgent reports can be issued.
Safe – Design of techniques in a closed reaction tube eliminates operator contact with reagents or other chemicals. In addition, the closed system with multiple controls reduces possible human errors.
Connected: The cobas® Liat® system offers bi-directional connectivity to integrate all equipment in “Point of Care” environments with a central data management system.
The Cobas Liat PCR System delivers definitive results through easy and intuitive user handling, bringing you from sample preparation to results with no operator interpretation required. To meet diagnostic and patient care needs, Roche is committed to developing a wide range of assays for the Cobas Liat system.
This currently includes the following parameters:
SARS-CoV-2 & Influenza A / B
C. difficile
Influenza A / B + RSV
CT / NG / MG *
Include:
Roche Diagnostics Cobas Liat Influenza A/B Assay
Roche Diagnostics Cobas Liat Strep A Control Kit
ROCHE DIAGNOSTICS CORPORATION COBAS LIAT SARSCOV2 KIT 20T/EA
ROCHE DIAGNOSTICS CORPORATION COBAS LIAT SARSCOV2 CTL 1KT/EA
Roche Diagnostics cobas™ Liat Cleaning Tool Kit
Power Cord for Cobas Liat PCR System Strep A Test Pack for Cobas Liat PCR System
Benefits at a glance
Quick results in 20 minutes or less2-8
cobas® liat benefit icon quick
Multi- and single-target assays with results in minutes
Less than one minute of hands-on time
Intuitive, easy-to-use interface with step-by-step guided instruction
No operator intervention or interpretation
No laptops or additional equipment required
Real-time PCR confidence
cobas® liat benefit icon confident
Gold standard PCR technology4-6
Reproducible4-6 results, wherever and whenever you need them
Accuracy at your fingertips, in all near-patient settings.
Designed for a fail-safe experience, the closed system reduces the risk of false results2-4
Strategically connected control
On-demand downloading of assay scripts and core software
Supporting enhanced cybersecurity by eliminating USB file transfer
Streamlined updates across networks of devices
Maintenance informed by real-world instrument data